Molecular biology professor Yibin Kang opened the doors to his lab in 2004. Since then, his lab has been researching cancer metastasis — the spread of cancers from a starting point to the rest of the body — using mouse models. In the Nov. 29 issue of Nature Cancer, his lab published not one, but two papers reporting an experimental treatment that has the potential to become the basis for a new cancer therapy.
“My lab focuses on a central question in cancer treatment: why some patients develop metastatic disease, and why they are so difficult to treat?” Kang wrote in an email to The Daily Princetonian.
“We try to identify the genes that make certain cancers particularly dangerous — growing rapidly, spreading to other parts of the body, evading the immune [system’s] surveillance and resistant to currently available treatment,” he explained.
One such gene, which Kang has been studying for more than 15 years, is called metadherin, or MTDH for short. It encodes a protein of the same name.
According to Kang, the outcomes for early-stage cancer patients can be starkly different, even for those receiving the same treatment. This pushed his research group to try and characterize genes that could potentially cause these differences. From a series of early experiments, they determined that MTDH was one such gene.
“Some patients are essentially cured after the surgery to remove the primary tumor and chemotherapy to prevent relapse,” he wrote in his email. “On the other hand, some other patients have recurrent diseases, often as metastatic cancer in distant organs, and they are usually very hard to treat, eventually leading to the death of the patients.”
MTDH was identified the same year Kang founded his research group at the University, and in 2009, his lab published a highly impactful paper that reported increased expression of the MTDH gene in 30 to 40 percent of tumor samples from breast cancer patients.
In other words, the MTDH gene was producing an increased amount of MTDH proteins in a portion of tumor samples. The accumulation of these proteins drives the spread of cancers, by allowing tumor cells to closely stick to blood vessels in faraway organs and make cancer cells more resistant to traditional chemotherapies.

Five years later, Kang’s group found that MTDH interacts with a protein called SND1 to maintain a specific shape. In biology, shape is essential to function, so the interaction between MTDH and SND1 is a key detail in understanding how MTDH functions to promote metastasis, and more importantly, how to target it.

The MTDH-SND1 complex promotes breast cancer progression and metastasis, and induces chemoresistance and immune evasion. Co-crystal structure showing a small molecule inhibitor (green) binding to a hydrophobic pocket on the surface of SND1, disrupting the interaction between MTDH and SND1 and enhancing treatment response of metastatic breast to chemotherapy and immunotherapy.
Kang’s lab has been trying to target MTDH in mice tumors to limit metastasis. Targeting MTDH is a particularly favorable potential approach, because according to the Kang lab’s findings, it’s not required for healthy development in mice. In other words, removing something that promotes cancer spread in a lot of human cancers, but isn’t required for general health, is an ideal solution.
In the two recent papers, the Kang lab reported multiple findings. In mice with metastatic breast cancer, they found that removing the MTDH gene — thereby preventing its expression — significantly slows down disease progression. They also identified a compound called C26A6, which can stop the interaction between MTDH and SND1. Because it can separate villain and sidekick, the molecule is promising.
“[We] achieved dramatic therapeutic response in metastatic cancers in mice, especially when combined with chemotherapy or immunotherapy,” Kang wrote.
Minhong Shen, an associate research scholar in the Kang lab, is the primary author of both papers.
In an email to the ‘Prince,’ he summarized the findings of the papers, writing, “We developed new small chemical compounds that could significantly enhance the chemotherapy and immunotherapy efficacy in breast cancer mouse models.”
“These studies open new avenues for developing therapies against metastatic breast cancer in [the] clinic,” he added.
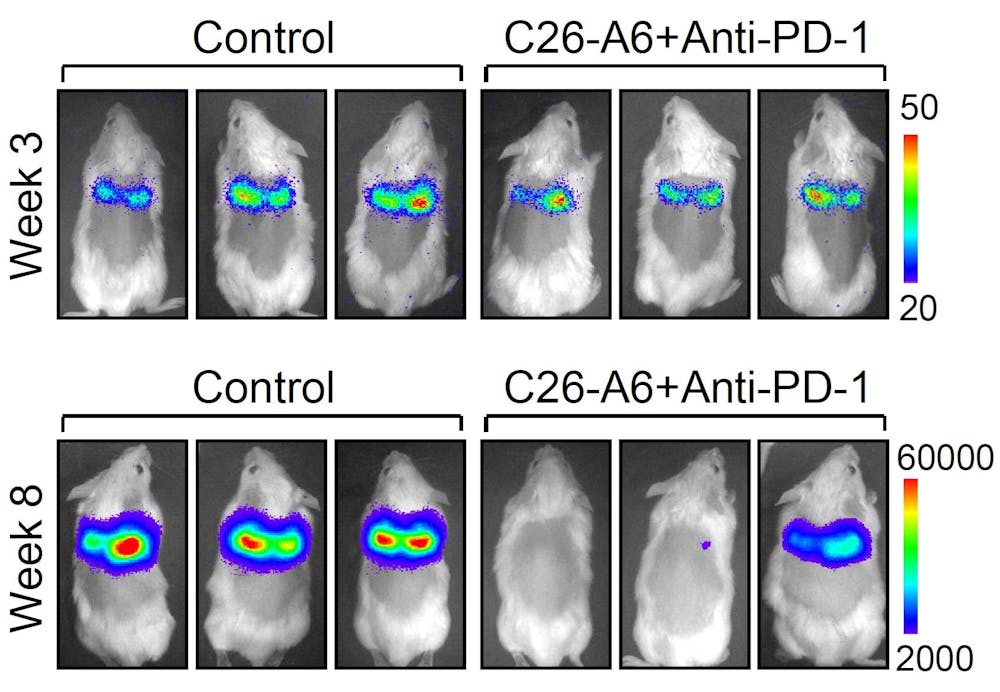
Mice with fully developed lung metastasis from breast cancer were treated with C26-A6 combined with anti-PD1 therapy, or with control vehicle. 5 weeks later, most mice responded to treatment with significantly reduction of lung metastasis burden, based on bioluminescence signals emitted from tumor cells.
The interaction between MTDH and SND1 is of particular importance, given that it is able to suppress immune system responses to developing tumors, based on the lab’s new findings. The interaction between these two proteins silences alarm bells in cancer cells; without hearing the sounding alarm, the immune system is unaware that it needs to rally against a growing enemy.
“[This mechanism] explains why tumors with high MTDH expression are so dangerous and difficult to treat,” Kang explained in his email.
The two newly published studies are a culmination of many years of research. Identifying MTDH as a gene of interest and developing a candidate drug that could target it happened through a series of steps. Studying the mechanisms behind cancer is a long, arduous process, as Kang pointed out.
“[The work] is equivalent to an ultramarathon in research. It really took years or even decades of persistent hard work to get to this point,” he emphasized.
Nearly 50,000 lives are lost to breast cancer each year in the US alone, Shen said. Both the metastasis itself, and lack of effective therapies are a cause — driving his team to continue running in this ‘ultramarathon.’
For Shen, his personal interactions with breast cancer patients, which regularly happen through the Kang lab, are what drive him to keep doing his work in the lab every day.
“From those conversations I understand how [depressed] people are when they get diagnosed with metastatic breast cancer, not only the patients themselves, but also their close relatives,” he wrote in his email. “That’s the fundamental source of encouragement to push me [to] focus on this research topic.”
“I hope my [studies] could benefit breast cancer patients in the future,” he added.
What are the next steps? To start moving from mice to humans. The two Nature Cancer studies show that C26A6 can stop cancer spread in mice. Now, the researchers hope to begin designing therapies around it that can hopefully be used to treat cancer in people.
“The drug candidate and technology platform has been licensed to a biotech startup that I [co-founded], and we are moving forward to develop this into effective medicine for human cancer patients,” Kang wrote.
Shen is hopeful for the future of the research.
“We are working with pharmaceutical companies to optimize the small chemical compounds we got,” he wrote. “Hopefully we will be able to push them into clinical trials soon.”
For the Kang lab, the ultramarathon that began over 15 years ago persists, as the researchers continue to seek to understand the underlying mechanisms behind cancer metastasis.
“Small-molecule inhibitors that disrupt the MTDH–SND1 complex suppress breast cancer progression and metastasis,” by Shen et al. and “Pharmacological disruption of the MTDH–SND1 complex enhances tumor antigen presentation and synergizes with anti-PD-1 therapy in metastatic breast cancer,” by Shen et al. both appear in the Nov. 29 issue of Nature Cancer.
Zoya Amir Gauhar is a senior writer who often covers research and the sciences. She can be reached at zgauhar@princeton.edu. She enjoys combining her science background and interviewing abilities to learn more about the people behind the research.








